Breast Cancer Risk Assessment Tool
Enter your information above to calculate your 5-year risk of breast cancer.
Exemestane is approved for breast cancer prevention in high-risk post-menopausal women with a 5-year risk ≥ 1.7%.
| Factor | Score | Description |
|---|---|---|
| Age (40-49) | 1.5 | Higher risk as age increases |
| Age (50-59) | 2.0 | Higher risk as age increases |
| Age (60-69) | 2.5 | Higher risk as age increases |
| Family history | 1.0 | One affected first-degree relative |
| Personal history | 2.0 | Atypical hyperplasia or LCIS |
| BRCA mutation | 3.0 | Significantly higher risk |
| Previous biopsy | 0.5 | Mildly increased risk |
When doctors talk about Exemestane is a steroidal aromatase inhibitor used to lower estrogen levels in post‑menopausal women, the goal isn’t just treating cancer - it’s stopping it before it starts. For women with a family history, BRCA mutations, or other risk factors, this drug offers a preventive option that’s gaining attention in the medical community.
Key Takeaways
- Exemestane blocks estrogen production, which fuels many breast cancers.
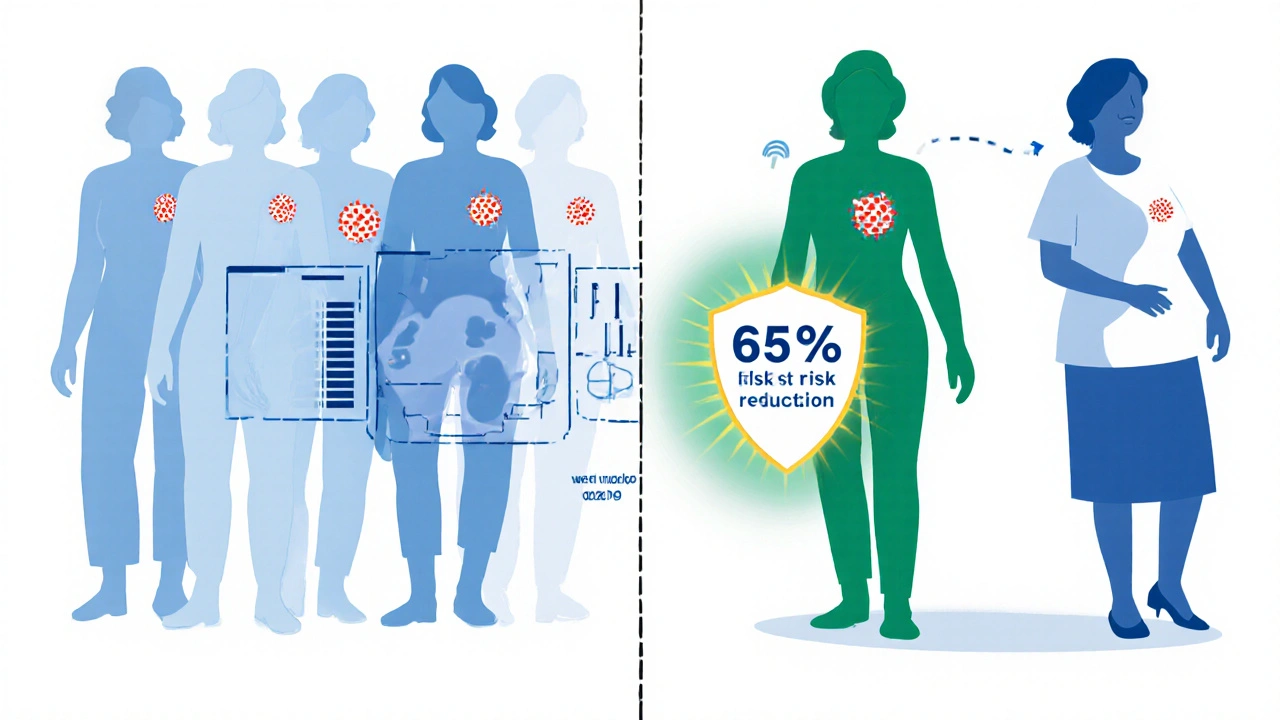
- Large trials (e.g., MAP.3) showed a 65% reduction in invasive breast cancer among high‑risk women.
- Compared with tamoxifen, exemestane has fewer uterine side effects but may affect bone density.
- Eligibility hinges on age, menopausal status, and a calculated 5‑year risk ≥ 1.7%.
- Regular monitoring of bone health and lipid profiles is essential during treatment.
How Exemestane Works
Exemestane belongs to the class of Aromatase inhibitors that permanently bind to the aromatase enzyme, preventing the conversion of androgens to estrogen. In post‑menopausal women, most estrogen is produced this way, so cutting it off starves hormone‑sensitive cells. The drug’s irreversible binding distinguishes it from non‑steroidal inhibitors like anastrozole, offering a slightly different side‑effect profile.
Evidence from Clinical Trials
The most compelling data come from the MAP.3 (IBIS‑2) trial, a double‑blind, placebo‑controlled study enrolling 4,560 women at increased risk of breast cancer. Participants took 25mg of exemestane daily for five years.
Results showed:
- 45 cases of invasive breast cancer in the exemestane group vs. 127 in the placebo group.
- A relative risk reduction of 65% (hazard ratio=0.35, 95%CI0.25‑0.49).
- No significant increase in cardiovascular events.
- Higher incidence of mild musculoskeletal aches and a modest drop in bone mineral density.
The trial earned FDA approval in 2014 for risk‑reduction use in post‑menopausal women with a 5‑year invasive breast cancer risk of at least 1.7%. Subsequent real‑world registries have confirmed similar efficacy, especially in women with prior atypical hyperplasia or lobular carcinoma in situ.
Comparing Exemestane with Other Chemopreventive Options
Historically, tamoxifen - a Selective estrogen receptor modulator (SERM) - was the go‑to drug for chemoprevention. While both lower breast‑cancer incidence, they differ in mechanism, side effects, and patient preference.
| Attribute | Exemestane | Tamoxifen |
|---|---|---|
| Class | Aromatase inhibitor | SERM |
| Mechanism | Blocks estrogen synthesis | Blocks estrogen receptors in breast tissue |
| Typical dose | 25mg oral daily | 20mg oral daily |
| 5‑year risk reduction (studies) | ≈65% (MAP.3) | ≈50% (NSABP P‑1) |
| Uterine cancer risk | None reported | Increased risk |
| Bone health impact | Potential loss; monitor BMD | Neutral or modest gain |
| Common side effects | Joint pain, hot flashes, mild bone loss | Hot flashes, vaginal dryness, thromboembolism |
| FDA-approved for prevention | Yes (post‑menopausal) | Yes (both pre‑ and post‑menopausal) |
Both drugs reduce risk, but the choice often hinges on a woman’s menopausal status, bone health, and tolerance for side effects. For a post‑menopausal woman with a modestly low bone density, tamoxifen might be preferable. Conversely, a woman with a uterus who wants to avoid the rare but serious uterine cancer risk may lean toward exemestane.
Who Should Consider Exemestane?
Eligibility isn’t a free‑for‑all. Physicians use models such as the Gail, Tyrer‑Cuzick, or BCRAT calculators to estimate a 5‑year invasive breast‑cancer risk. Generally, a woman qualifies if she meets all of the following:
- Post‑menopausal status (no menstrual periods for ≥12months).
- 5‑year risk ≥1.7% according to a validated risk model.
- History of atypical hyperplasia, lobular carcinoma in situ, or a known BRCA1/2 mutation.
- No prior diagnosis of invasive breast cancer.
- Acceptable baseline bone mineral density (T‑score >‑2.0) or willingness to take calcium/vitaminD and possibly bisphosphonates.
Patients with severe liver disease, uncontrolled osteoporosis, or a history of thromboembolic events should discuss alternatives with their oncologist.

Benefits and Risks in Plain Terms
Benefits:
- Significant drop in the chance of developing invasive breast cancer.
- Oral once‑daily dosing - easy to incorporate into daily routine.
- No increased risk of uterine cancer, unlike tamoxifen.
Risks:
- Potential bone loss; regular DEXA scans recommended.
- Joint and muscle aches, especially during the first year.
- Hot flashes and mild fatigue are common.
- Rarely, liver enzyme elevations - labs are checked at baseline and periodically.
Most side effects are manageable with lifestyle tweaks and supportive medications (e.g., NSAIDs for joint pain, bisphosphonates for bone protection).
Practical Guidance for Starting Exemestane
- Confirm post‑menopausal status and calculate 5‑year risk using a validated tool.
- Obtain baseline labs: liver function, lipid panel, and a DEXA scan for bone density.
- Prescribe 25mg orally each morning with food to reduce stomach upset.
- Schedule follow‑up visits every six months to monitor liver enzymes, lipid changes, and any new symptoms.
- Repeat DEXA at 1‑year and then every two years; add calcium (1,200mg) and vitaminD (800-1,000IU) daily.
- Consider adding a bisphosphonate (e.g., alendronate 70mg weekly) if T‑score drops below‑2.0.
- Encourage regular weight‑bearing exercise (30minutes, 3‑5 times/week) to support bone health.
- Discontinue after five years of therapy or earlier if intolerable side effects develop; discuss post‑treatment surveillance with the oncologist.
Frequently Asked Questions
Can pre‑menopausal women take exemestane for prevention?
No. Exemestane’s mechanism requires low baseline estrogen, which only occurs after menopause. Pre‑menopausal women typically use tamoxifen or consider clinical trials.
How does exemestane affect cholesterol?
A modest increase in LDL cholesterol has been observed in up to 10% of users. Routine lipid panels are recommended, and statin therapy may be initiated if levels rise significantly.
Is the bone loss caused by exemestane permanent?
When bone density is monitored and protective measures (calcium, vitaminD, bisphosphonates) are employed, loss is usually reversible after stopping the drug. Continuous follow‑up is key.
What is the typical duration of chemoprevention with exemexane?
The FDA label recommends a five‑year course, mirroring the duration used in the MAP.3 trial. Some clinicians may extend therapy based on ongoing risk assessment.
Can exemestane be taken with other hormone‑related medications?
Concurrent use of estrogen‑containing hormones (like hormone‑replacement therapy) defeats the purpose of aromatase inhibition and is contraindicated. Discuss any supplements with your doctor.
For anyone at heightened risk, the decision to start a preventive drug is personal and should be made alongside a knowledgeable healthcare provider. With the right monitoring plan, exemestane can be a powerful tool in the fight against breast cancer.



Lila Tyas
October 15, 2025 AT 18:09Wow, this is such a game‑changer for women at risk! 🎉 Exemestane’s ability to slash that breast‑cancer risk by almost two‑thirds is amazing. If you’re post‑menopausal and meet the criteria, this could be your ticket to peace of mind. Don’t forget the bone health checks – a little calcium and weight‑bearing exercise go a long way. Talk to your doc and see if it’s right for you!
Mark Szwarc
October 26, 2025 AT 07:33Let’s break down the key points. Exemestane targets aromatase, permanently lowering estrogen synthesis, which directly impacts hormone‑driven tumors. The MAP‑3 data show a 65 % relative risk reduction, a figure that outperforms tamoxifen in many sub‑analyses. However, clinicians must monitor bone mineral density and lipid panels throughout the five‑year course. Side effects such as joint pain are common but manageable with NSAIDs or dose adjustments. In short, for eligible post‑menopausal patients, the benefit‑risk profile is compelling.
BLAKE LUND
November 5, 2025 AT 21:57In the grand theater of prevention, exemestane pirouettes gracefully, stealing estrogen’s spotlight while whispering sweet nothings to rogue cells.
Veronica Rodriguez
November 16, 2025 AT 12:21Here’s a quick cheat‑sheet: start with a baseline DEXA, keep calcium at 1,200 mg and vitamin D at 1,000 IU, and schedule labs every six months 😊. If bone loss appears, a bisphosphonate can reverse the trend. Keep an eye on LDL – a statin may be needed. Most patients tolerate the drug well, and the reduction in invasive cancer is worth the monitoring.
Holly Hayes
November 27, 2025 AT 02:45Honestly, the literature on exemestane is, like, sooo elite – only the savviest oncologists even attempt to decipher the 65% risk‑reduction stat. If ur bone density ain’t up to snuff, maybe consider tamoxifen. The whole aromatase‑inhibitor drama is just another layer of the pharma circus, don’t ya think?
Matthew Shapiro
December 7, 2025 AT 17:09Exemestane provides a solid option for chemoprevention, especially when uterine safety is a priority. The requirement for adequate baseline bone density is crucial, so a DEXA scan is non‑negotiable. Monitoring liver enzymes and lipid profiles keeps the regimen safe. For patients who can tolerate mild arthralgia, the five‑year course aligns with the MAP‑3 protocol. Overall, it’s a reasonable trade‑off for many high‑risk women.
Julia Phillips
December 18, 2025 AT 07:33Imagine standing at a crossroads, the shadow of breast cancer looming, and then being handed a key that could lock that door – that’s what exemestane offers to those brave souls who’ve faced family tragedy. The statistics speak volumes, yet each number represents a mother, a sister, a daughter who might breathe easier. While the bone health concerns are real, they can be softened with proper nutrition and gentle exercise. Let’s celebrate the science that gives us hope, and honor the women who choose this path with quiet determination.
Robert Keter
December 28, 2025 AT 21:57Exemestane’s mechanism of action is elegantly simple yet profoundly impactful, as it irreversibly binds to the aromatase enzyme, halting the conversion of androgens to estrogen in post‑menopausal women. This biochemical blockade directly starves hormone‑dependent tumor cells of the estrogenic signal they crave for proliferation. The MAP‑3 trial, a landmark double‑blind, placebo‑controlled study, enrolled over four thousand high‑risk participants and demonstrated a striking 65 % reduction in invasive breast cancer incidence. Such a reduction surpasses many of the older chemoprevention agents, positioning exemetane as a front‑line preventive therapy for suitable patients. However, the benefits are accompanied by a notable side‑effect profile that mandates vigilant monitoring. Bone mineral density, for instance, can modestly decline over the five‑year treatment period, prompting clinicians to incorporate DEXA scans at baseline and periodic intervals. Calcium and vitamin D supplementation, coupled with weight‑bearing exercise, serve as first‑line countermeasures against osteopenia. In cases where BMD falls below a T‑score of ‑2.0, the addition of bisphosphonates such as alendronate can arrest or even reverse bone loss. Musculoskeletal aches, another common complaint, often respond to over‑the‑counter NSAIDs or dose timing adjustments, particularly taking the pill with food to minimize gastrointestinal discomfort. Lipid alterations, specifically a modest rise in LDL cholesterol, have been reported in roughly ten percent of users, underscoring the importance of routine lipid panels and potential statin therapy. Liver function tests should also be performed at baseline and semi‑annually, as rare elevations in transaminases have been observed. Importantly, exemestane does not confer an increased risk of uterine malignancy, differentiating it from tamoxifen and making it an attractive option for women with an intact uterus. Psychological tolerability is another consideration; hot flashes and fatigue are noted but generally manageable with lifestyle modifications. Overall, the decision to embark on a five‑year course of exemestane should be a shared, informed choice between patient and provider, weighing the substantial cancer risk reduction against the manageable, yet non‑trivial, side‑effect spectrum. Regular follow‑up visits, diligent laboratory surveillance, and a proactive bone health strategy collectively ensure that the therapeutic advantages of exemestane are maximized while mitigating its risks.
Rory Martin
January 8, 2026 AT 12:21It must be noted, dear readers, that the promotion of exemestane by mainstream oncological societies aligns suspiciously with pharmaceutical interests that seek to expand market share under the guise of preventive care. One cannot ignore the subtle pressure exerted on clinicians to adopt this regimen without a thorough public discourse on long‑term ramifications. The data, while statistically impressive, are curated, and the underlying mechanisms of bone loss remain insufficiently disclosed. Consequently, an informed skeptic should demand greater transparency before embracing such a protocol.
Maddie Wagner
January 19, 2026 AT 02:45Let’s lift each other up as we explore this preventive option – every woman’s risk profile is unique, and the decision to start exemestane should feel empowering, not isolating. Remember, you’re not alone on this journey; a supportive network of healthcare providers, family, and peers can make the monitoring process smoother. Embrace the strength that comes from knowledge, and celebrate the courage it takes to take proactive steps. Together we can rewrite the narrative around breast‑cancer risk.
Boston Farm to School
January 29, 2026 AT 17:09exemestane works by shutting down aromatase so estrogen drops and risk goes down 😐 regular bone scans are a must keep labs on schedule and talk to your doc about calcium vitamin d and possible bisphosphonates