Most people think side effects from medications are just bad luck - something random that happens when your body doesn’t react the way it should. But that’s not true. Side effects aren’t accidents. They’re predictable results of how drugs interact with your body at a molecular level. And understanding the difference between on-target and off-target effects changes everything about how we think about drug safety, effectiveness, and even why some drugs get repurposed entirely.
What Exactly Are On-Target Effects?
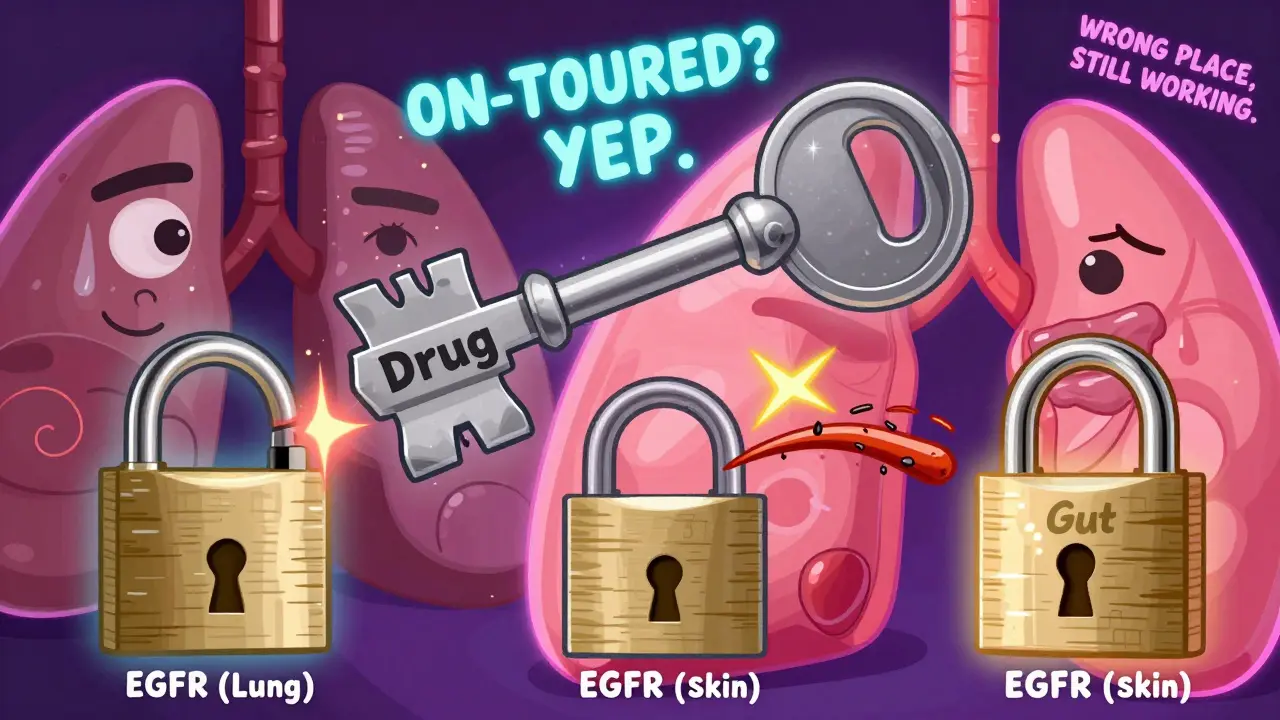
On-target effects happen when a drug does exactly what it was designed to do - but in the wrong place or too strongly. Think of it like a key that fits perfectly into a lock (the target), but turns that lock in places it wasn’t supposed to. For example, drugs that block the EGFR protein to treat lung cancer also block EGFR in your skin. That’s why so many patients develop painful rashes. It’s not a mistake. It’s the drug working exactly as intended - just in a tissue that wasn’t the target of treatment.
Another classic case is metformin, a diabetes drug. It lowers blood sugar by reducing liver glucose production. But it also acts on the gut, slowing digestion and changing gut bacteria. That’s why diarrhea is so common. It’s not a side effect in the sense of being unwanted - it’s the same mechanism working in a different organ. Doctors call these "expected" side effects because they’re well-documented, predictable, and often manageable.
According to a 2021 survey of over 1,200 physicians published in JAMA Internal Medicine, 82% of doctors consider on-target side effects "expected and manageable." They’re frustrating, yes - but not surprising. That’s why oncologists routinely monitor skin rashes from EGFR inhibitors or joint pain from arthritis drugs that affect immune pathways. These aren’t failures. They’re signals that the drug is hitting its mark.
What Are Off-Target Effects - And Why Are They More Dangerous?
Off-target effects are the real wild cards. These happen when a drug binds to something it wasn’t meant to. A molecule designed to block one protein accidentally sticks to another - maybe because the two proteins look similar, or because the drug is chemically "sticky" and grabs anything nearby. These interactions are often unpredictable, poorly understood, and can cause serious harm.
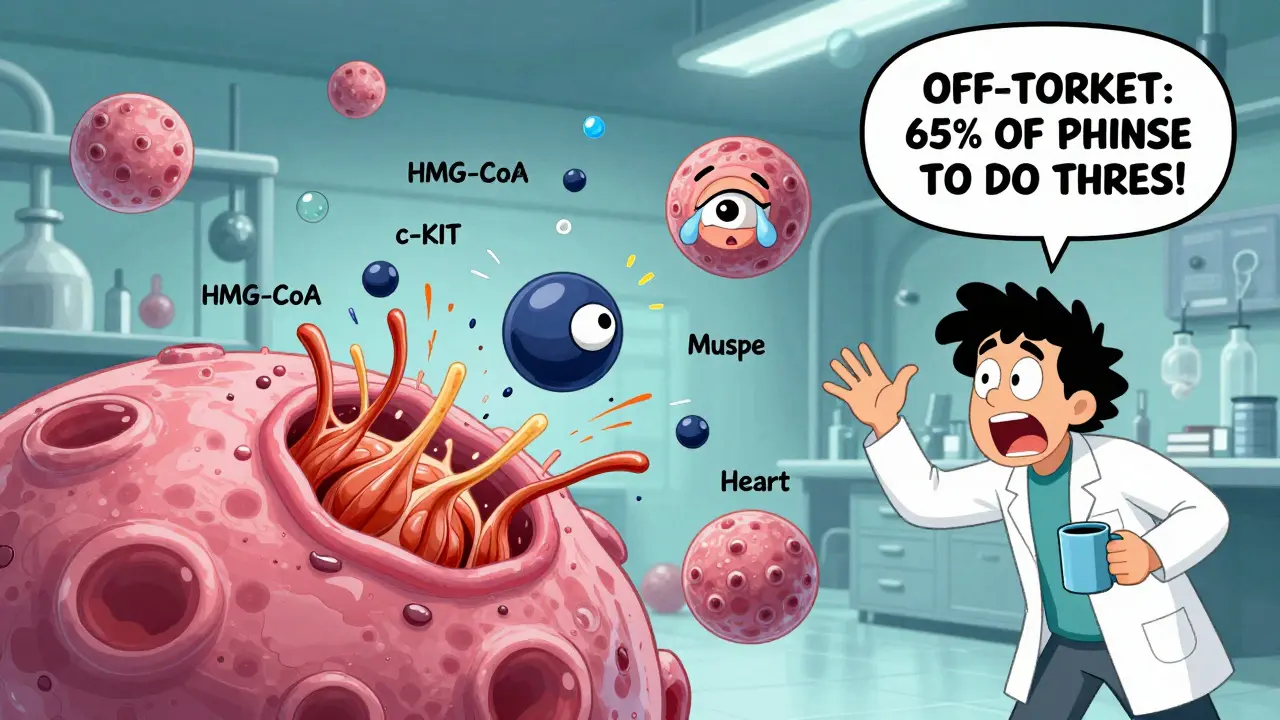
Take statins, the cholesterol-lowering drugs millions take daily. Their main job is to block HMG-CoA reductase in the liver. But they also affect muscle cells. In some people, this leads to rhabdomyolysis - a dangerous breakdown of muscle tissue. That’s an off-target effect. The drug didn’t "fail" at its job. It just did another job it wasn’t supposed to.
Kinase inhibitors - a class of cancer drugs - are especially notorious. A 2017 study in Nature Chemical Biology found that most kinase inhibitors bind to 25-30 different kinases at therapeutic doses. Imatinib (Gleevec), for example, works brilliantly against chronic myeloid leukemia by blocking the BCR-ABL protein. But it also hits c-KIT, which helps explain why some patients get swelling in their legs or around their eyes. That’s not a flaw in the patient. It’s a flaw in the drug’s selectivity.
The FDA found that off-target effects account for 65% of drug failures in Phase II trials between 2015 and 2020. That’s not small. It’s the leading reason drugs get pulled from development after millions are spent. And here’s the kicker: we used to think these were rare. Now we know that 60-80% of all small-molecule drugs interact with multiple targets. Most of them just weren’t tested well enough before being approved.
Why Some Off-Target Effects Become Treatments
Not all off-target effects are bad. Sometimes, they’re golden.
Thalidomide was pulled from the market in the 1960s after causing severe birth defects - a devastating off-target effect. Decades later, researchers discovered it had powerful immunomodulatory properties. Today, it’s a standard treatment for multiple myeloma. The same molecule, the same side effect, repurposed.
Sildenafil (Viagra) was originally developed as a heart medication. It was meant to relax blood vessels in the heart. But during trials, men reported something else: erections. Turns out, the drug also blocked phosphodiesterase-5 in penile tissue. That was an off-target effect. Now, it’s one of the most successful drugs ever made.
Even chloroquine, once touted as a COVID-19 treatment, likely worked through off-target effects - not by killing the virus directly, but by changing how cells handle acid. That’s why it failed in large trials: it wasn’t hitting the right target. But its off-target action was real. It just wasn’t the one we hoped for.
This isn’t luck. It’s biology. Drugs don’t always know what they’re doing. And sometimes, what they do accidentally turns out to be more useful than what they were designed to do.
Why Biologics Have Fewer Off-Target Problems
Not all drugs are created equal. Small molecules - pills and capsules - are simple, portable, and cheap to make. But they’re also chemically "noisy." They’re like a hammer: effective, but blunt. They can hit multiple targets because they’re small enough to slip into places they shouldn’t.
Biologics - like monoclonal antibodies (think Herceptin or Keytruda) - are much larger, more precise molecules. They’re designed to fit like a lock-and-key into one specific protein on a cell surface. As a result, they’re far more selective. A 2018 review in Nature Reviews Drug Discovery found that small molecules average 6.3 off-target interactions at therapeutic doses. Biologics? Just 1.2.
That’s why Herceptin, which targets HER2 in breast cancer, has fewer unpredictable side effects than a kinase inhibitor. But it’s not perfect. Even biologics can cause on-target toxicity. Herceptin can weaken heart muscle because HER2 is also important for heart cell repair. That’s why doctors monitor heart function during treatment. The effect is still on-target - just in a vital organ.
How Scientists Find Off-Target Effects Before It’s Too Late
Back in the 1990s, drug companies focused on one thing: finding a target and hitting it hard. They assumed specificity meant safety. That changed after dozens of drugs failed in late-stage trials.
Today, companies use multi-layered screening. One method, called chemical proteomics, uses drug molecules attached to beads to fish out all the proteins they bind to in a cell. Another, called transcriptome analysis, looks at how gene expression changes after drug exposure. If a drug changes the same genes as a genetic knockout of its target, that’s likely on-target. If it changes other genes - especially ones linked to inflammation or immune response - that’s off-target.
A 2019 study in Nature Scientific Reports tested four different statins across three cell types. At the gene level, each statin had a unique signature. No two were the same. But when they looked at pathways - the bigger biological networks - they found consistent patterns. All statins affected cholesterol metabolism (on-target). But only some triggered immune responses (off-target). That’s the key insight: gene-level noise fades when you zoom out to pathways.
Now, companies like Genentech and Novartis use AI to predict off-target binding based on chemical structure. The Open Targets Platform, used by 87% of top pharma firms, combines genomic, chemical, and clinical data to flag risky interactions before a drug even reaches humans.

What This Means for Patients
If you’re taking a drug and get a side effect, ask: Is this the drug working too hard, or is it doing something it shouldn’t?
On-target effects - like diarrhea from metformin, rash from EGFR inhibitors, or fatigue from thyroid drugs - are usually manageable. Dose adjustments, supportive care, and time often help. They’re part of the trade-off.
Off-target effects - like sudden muscle pain from statins, liver damage from antibiotics, or unexplained heart rhythm changes - are red flags. They’re unpredictable, sometimes irreversible, and often require stopping the drug. That’s why doctors run blood tests, monitor symptoms closely, and sometimes switch medications entirely.
One Reddit user summed it up perfectly: "I didn’t realize the diarrhea from my diabetes medication was actually the intended effect working too well in my gut." That’s on-target. But if your legs swell after taking a blood pressure pill, and you’ve never had swelling before? That might be off-target. And that’s worth talking to your doctor about.
The Future: Smarter Drugs, Fewer Surprises
The pharmaceutical industry is shifting. Companies are moving away from pure "target-based" drug design. Instead, they’re using phenotypic screening - testing compounds in whole cells or even whole organisms to see what they do, not just what they’re supposed to do.
Why? Because biology is messy. A drug that works beautifully in a test tube might be useless in a human. A drug that hits five targets might be more effective than one that hits one perfectly. Dr. Ben Cravatt at Scripps Research says phenotypic screening finds drugs with better safety profiles because they naturally balance efficacy and side effects.
By 2030, experts predict that drugs with fully mapped on-target and off-target profiles will control 78% of the global pharmaceutical market. That’s because precision medicine isn’t just about matching drugs to genes - it’s about matching drugs to people based on how their bodies react to every molecular interaction.
And it’s not just about avoiding harm. It’s about discovering new uses. The next great drug might come from an old one - the one you thought was too risky to keep using.
Are all side effects caused by off-target effects?
No. Many side effects are on-target - meaning the drug is working exactly as intended, just in the wrong place. For example, metformin causes diarrhea because it affects the gut, which is part of its mechanism for lowering blood sugar. Off-target effects are unintended interactions with other biological targets, like when a statin accidentally damages muscle cells.
Can a drug have both on-target and off-target effects at the same time?
Absolutely. Most drugs do. Imatinib (Gleevec) blocks BCR-ABL to treat leukemia (on-target), but it also inhibits c-KIT, which causes swelling and fluid retention (off-target). Many cancer drugs work this way - the benefit comes from one action, and the side effect from another.
Why do some people get side effects and others don’t?
Genetics, metabolism, age, and even gut bacteria play a role. Some people have genetic variants that make them more sensitive to a drug’s off-target binding. Others metabolize drugs slower, leading to higher exposure. That’s why two people on the same dose can have completely different reactions.
Are off-target effects always dangerous?
Not always. Some off-target effects led to blockbuster drugs. Sildenafil (Viagra) was originally for heart disease - its erectile side effect became its main use. Thalidomide’s teratogenic effect was tragic, but its immune-modulating action now treats multiple myeloma. Off-target doesn’t always mean bad - just unintended.
How do drug companies test for off-target effects today?
They use a mix of methods: chemical proteomics to find what proteins a drug binds to, transcriptomics to see how gene expression changes, and AI models that predict binding based on chemical structure. Platforms like Open Targets integrate data from thousands of studies to flag risky interactions before human trials begin.
Why are kinase inhibitors so prone to off-target effects?
Kinase proteins are structurally very similar - they all have a similar pocket where drugs bind. A drug designed to block one kinase often slips into others. Studies show kinase inhibitors bind to an average of 25-30 different kinases at therapeutic doses. That’s why they’re the most common source of off-target toxicity reports.

Robert Shiu
February 20, 2026 AT 20:04Man, this post nailed it. I’ve been on metformin for years and thought my constant diarrhea was just bad luck. Turns out? My gut’s just doing its job too well. No wonder my doctor never seemed worried about it - it’s not a side effect, it’s the drug working. Kinda wild to think our bodies are just along for the ride while these molecules go full ninja on our systems.
Also, I never realized statins could mess with muscles like that. My uncle had to quit his because of cramps so bad he couldn’t walk. Now I get why. Not a flaw in him - a flaw in the molecule. We need way more transparency on this stuff.
And hey - if thalidomide and Viagra came from "mistakes," maybe we’re throwing away good drugs too fast. Pharma’s too scared of lawsuits to explore the weird side effects. We’re losing potential cures because we’re too busy saying "nope, that’s bad."